Receptores de membrana GABRA5 são alvos moleculares na identificação de gliomas?
Conteúdo do artigo principal
Resumo
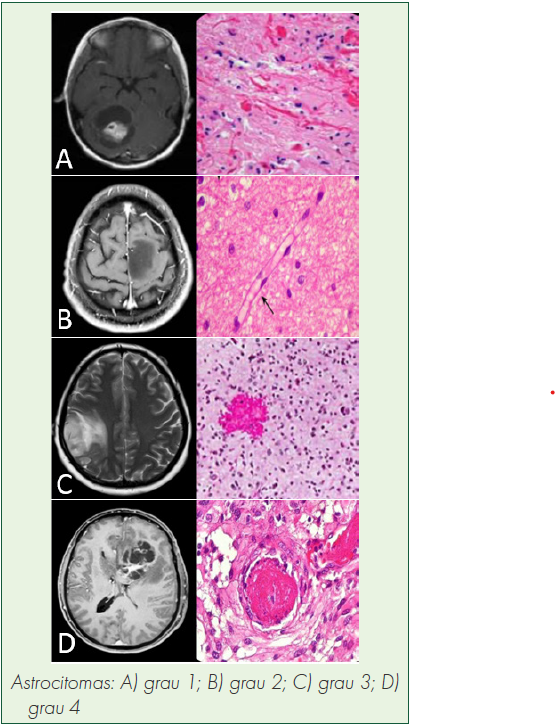
Introdução: Gliomas são os tumores primários mais comuns do sistema nervoso central e a maioria é formada por glioblastomas, com graus elevados de proliferação e invasão, com heterogeneidade biológica e pouca resposta aos tratamentos atuais. Imunoterapia e geneterapia não têm mostrado eficácia e, por isso, é necessário buscar conhecimento de novas moléculas e genes envolvidos na sua carcinogênese.
Objetivos: Avaliar se o receptor do ácido gama-aminobutírico alfa 5 (GABRA5) é potencial alvo terapêutico ou biomarcador para gliomas de diferentes subgrupos, e a correlação entre os níveis de ácido ribonucleico mensageiro (mRNA) de GABRA5 com a sobrevida global.
Métodos: Revisão integrativa feita em plataformas virtuais, em português e inglês, realizada por descritores relacionados ao tema, descritos no DeCS como “glioma, sistema nervoso central, glioblastoma” com busca AND ou OR, considerando-se inicialmente o título e/ou resumo. Após, naqueles que tinham maior relação ao tema, foi realizada a leitura na íntegra dos textos.
Resultados: Foram incluídos 36 artigos.
Conclusão: Os níveis de mRNA de GABRA5 em amostras de gliomas de todos os subgrupos histológicos são menores, em comparação ao controle. Não foi observada correlação entre os níveis de mRNA de GABRA5 com a sobrevida global dos subgrupos avaliados.
Detalhes do artigo

Este trabalho está licenciado sob uma licença Creative Commons Attribution 4.0 International License.
Referências
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97-109. Doi: 10.1007/s00401-007-0243-4.
Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol. 2005;109(1):93-108. Doi: 10.1007/s00401-005-0991-y.
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-96. Doi: 10.1056/NEJMoa043330.
Soffietti R, Baumert BG, Bello L, Von Deimling A, Duffau H, Frénay M, et al. Guidelines on management of low-grade gliomas: report of an EFNS-EANO Task Force. Eur J Neurol. 2010;17(9):1124-33. Doi: 10.1111/j.1468-1331.2010.03151.x.
Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev. 2008;60(3):243-60. Doi: 10.1124/pr.108.00505.
Louis DN. WHO classification of tumours of the central nervous system. 2016.
Chang EF, Smith JS, Chang SM, Lamborn KR, Prados MD, Butowski N, et al. Preoperative prognostic classification system for hemispheric low-grade gliomas in adults. J Neurosurg. 2008;109(5):817-24. Doi: 10.3171/JNS/2008/109/11/0817.
Yang P, Zhang W, Wang Y, Peng X, Chen B, Qiu X, et al. IDH mutation and MGMT promoter methylation in glioblastoma: results of a prospective registry. Oncotarget. 2015;6(38):40896-906. Doi: 10.18632/oncotarget.5683.
Braat S, Kooy RF. The GABAA Receptor as a Therapeutic Target for Neurodevelopmental Disorders. Neuron. 2015;86(5):1119-30. Doi: 10.1016/j.neuron.2015.03.042.
Kallay L, Keskin H, Ross A, Rupji M, Moody OA, Wang X, et al. Modulating native GABA. J Neurooncol. 2019;142(3):411-22. Doi: 10.1007/s11060-019-03115-0.
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021;23(8):1231-51. Doi: 10.1093/neuonc/noab106.
Bailey P, Cushing H. A classification of the tumors of the glioma group on a histogenetic basis with a correlated study of prognosis. Philadelphia: JB Lippincott; 1926.
Louis DN, Giannini C, Capper D, Paulus W, Figarella-Branger D, Lopes MB, et al. cIMPACT-NOW update 2: diagnostic clarifications for diffuse midline glioma, H3 K27M-mutant and diffuse astrocytoma/anaplastic astrocytoma, IDH-mutant. Acta Neuropathol. 2018;135(4):639-42. Doi: 10.1007/s00401-018-1826-y.
Brat DJ, Aldape K, Colman H, Holland EC, Louis DN, Jenkins RB, et al. cIMPACT-NOW update 3: recommended diagnostic criteria for "Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV". Acta Neuropathol. 2018;136(5):805-10. Doi: 10.1007/s00401-018-1913-0.
Louis DN, Wesseling P, Paulus W, Giannini C, Batchelor TT, Cairncross JG, et al. cIMPACT-NOW update 1: Not Otherwise Specified (NOS) and Not Elsewhere Classified (NEC). Acta Neuropathol. 2018;135(3):481-4. Doi: 10.1007/s00401-018-1808-0.
Ellison DW, Aldape KD, Capper D, Fouladi M, Gilbert MR, Gilbertson RJ, et al. cIMPACT-NOW update 7: advancing the molecular classification of ependymal tumors. Brain Pathol. 2020;30(5):863-6. Doi: 10.1111/bpa.12866.
Ellison DW, Hawkins C, Jones DTW, Onar-Thomas A, Pfister SM, Reifenberger G, et al. cIMPACT-NOW update 4: diffuse gliomas characterized by MYB, MYBL1, or FGFR1 alterations or BRAF. Acta Neuropathol. 2019;137(4):683-7. Doi: 10.1007/s00401-019-01987-0.
Brat DJ, Aldape K, Colman H, Figrarella-Branger D, Fuller GN, Giannini C, et al. cIMPACT-NOW update 5: recommended grading criteria and terminologies for IDH-mutant astrocytomas. Acta Neuropathol. 2020;139(3):603-8. Doi: 10.1007/s00401-020-02127-9.
Louis DN, Wesseling P, Aldape K, Brat DJ, Capper D, Cree IA, et al. cIMPACT-NOW update 6: new entity and diagnostic principle recommendations of the cIMPACT-Utrecht meeting on future CNS tumor classification and grading. Brain Pathol. 2020;30(4):844-56. Doi: 10.1111/bpa.12832.
Louis DN, Perry A, Burger P, Ellison DW, Reifenberger G, von Deimling A, et al. International Society Of Neuropathology--Haarlem consensus guidelines for nervous system tumor classification and grading. Brain Pathol. 2014;24(5):429-35. Doi: 10.1111/bpa.12171.
Reuss DE, Mamatjan Y, Schrimpf D, Capper D, Hovestadt V, Kratz A, et al. IDH mutant diffuse and anaplastic astrocytomas have similar age at presentation and little difference in survival: a grading problem for WHO. Acta Neuropathol. 2015;129(6):867-73. Doi: 10.1007/s00401-015-1438-8.
Pajtler KW, Witt H, Sill M, Jones DT, Hovestadt V, Kratochwil F, et al. Molecular Classification of Ependymal Tumors across All CNS Compartments, Histopathological Grades, and Age Groups. Cancer Cell. 2015;27(5):728-43. Doi: 10.1016/j.ccell.2015.04.002.
Andreiuolo F, Varlet P, Tauziède-Espariat A, Jünger ST, Dörner E, Dreschmann V, et al. Childhood supratentorial ependymomas with YAP1-MAMLD1 fusion: an entity with characteristic clinical, radiological, cytogenetic and histopathological features. Brain Pathol. 2019;29(2):205-16. Doi: 10.1111/bpa.12659.
von Deimling A, Ono T, Shirahata M, Louis DN. Grading of Diffuse Astrocytic Gliomas: A Review of Studies Before and After the Advent of IDH Testing. Semin Neurol. 2018;38(1):19-23. Doi: 10.1055/s-0038-1636430.
Shirahata M, Ono T, Stichel D, Schrimpf D, Reuss DE, Sahm F, et al. Novel, improved grading system(s) for IDH-mutant astrocytic gliomas. Acta Neuropathol. 2018;136(1):153-66. Doi: 10.1007/s00401-018-1849-4.
Cimino PJ, Holland EC. Targeted copy number analysis outperforms histologic grading in predicting patient survival for WHO grades II/III IDH-mutant astrocytomas. Neuro Oncol. 2019;21(6):819-21. Doi: 10.1093/neuonc/noz052.
Nobusawa S, Watanabe T, Kleihues P, Ohgaki H. IDH1 mutations as molecular signature and predictive factor of secondary glioblastomas. Clin Cancer Res. 2009;15(19):6002-7. Doi: 10.1158/1078-0432.CCR-09-0715.
Jain SU, Do TJ, Lund PJ, Rashoff AQ, Diehl KL, Cieslik M, et al. PFA ependymoma-associated protein EZHIP inhibits PRC2 activity through a H3 K27M-like mechanism. Nat Commun. 2019;10(1):2146. Doi: 10.1038/s41467-019-09981-6.
Lucas CG, Mueller S, Reddy A, Taylor JW, Oberheim Bush NA, Clarke JL, et al. Diffuse hemispheric glioma, H3 G34-mutant: Genomic landscape of a new tumor entity and prospects for targeted therapy. Neuro Oncol. 2021;23(11):1974-6. Doi: 10.1093/neuonc/noab184.
Synowitz M, Ahmann P, Matyash M, Kuhn SA, Hofmann B, Zimmer C, et al. GABA(A)-receptor expression in glioma cells is triggered by contact with neuronal cells. Eur J Neurosci. 2001;14(8):1294-302. Doi: 10.1046/j.0953-816x.2001.01764.x.
Everington EA, Gibbard AG, Swinny JD, Seifi M. Molecular Characterization of GABA-A Receptor Subunit Diversity within Major Peripheral Organs and Their Plasticity in Response to Early Life Psychosocial Stress. Front Mol Neurosci. 2018;11:18. Doi: 10.3389/fnmol.2018.00018.
Olsen RW. GABA. Neuropharmacology. 2018;136(Pt A):10-22. Doi: 10.1016/j.neuropharm.2018.01.036.
Ravasz D, Kacso G, Fodor V, Horvath K, Adam-Vizi V, Chinopoulos C. Catabolism of GABA, succinic semialdehyde or gamma-hydroxybutyrate through the GABA shunt impair mitochondrial substrate-level phosphorylation. Neurochem Int. 2017;109:41-53. Doi: 10.1016/j.neuint.2017.03.008.
Ghit A, Assal D, Al-Shami AS, Hussein DEE. GABA. J Genet Eng Biotechnol. 2021;19(1):123. Epub 20210821. Doi: 10.1186/s43141-021-00224-0.
Jembrek MJ, Vlainic J. GABA Receptors: Pharmacological Potential and Pitfalls. Curr Pharm Des. 2015;21(34):4943-59. Doi: 10.2174/1381612821666150914121624.
Matuszek M, Jesipowicz M, Kleinrok Z. GABA content and GAD activity in gastric cancer. Med Sci Monit. 2001;7(3):377-81.
Volski IM, de Andrade L, Costa RS, Volc SM, dos Santos ZFDG. Necessidades de saúde dos adolescentes e adultos jovens com câncer. 2022;80(2):54-7. Doi: 10.55684/80.2.13
Buffon VA, Conti BP, Beltrame CM, Sobral ACL, Simm EB, Bark SA. Perfil epidemiológico de tumores intracranianos metastáticos submetidos à neurocirurgia. BioSCIENCE. 2022;80(2):100-5. Doi: 10.55684/80.2.23