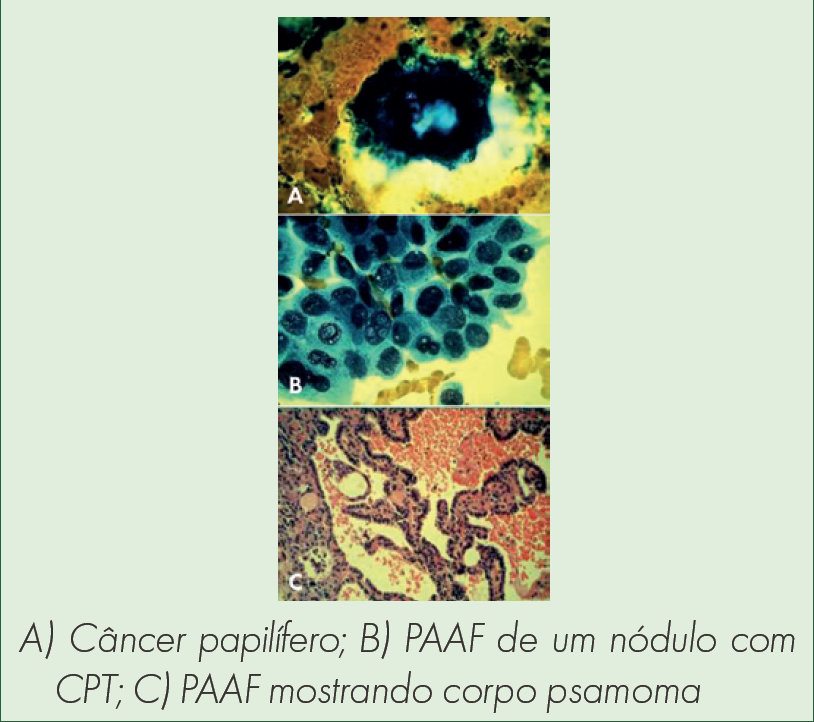
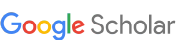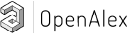Como a Ciclina D1 se comporta como biomarcador nos carcinomas papilíferos de tireoide e bócios multinodulares?
Conteúdo do artigo principal
Resumo
Racional: Os carcinomas papilíferos são os mais prevalentes e menos agressivos de tireoide (CPT). Em alguns casos, o diagnóstico é duvidoso e o prognóstico ruim. A busca de biomarcadores teciduais que permitam assegurar tanto o diagnóstico para casos indeterminados, quanto o prognóstico, identificando os casos de maior agressividade, têm sido estudadas nas últimas décadas.
Objetivo: Revisar na literatura na busca da ciclina D1 como marcador dos carcinomas papilíferos de tireoide e nos bócios multinodulares, e avaliar se a expressão dela apresenta correlação com as características clínicopatológicas dos carcinomas papilíferos de tireoide.
Métodos: Revisão narrativa feita colhendo informações para leitura e análise a partir de pesquisa online em platoformas virtuais. Inicialmente foi realizada busca por descritores DECs relacionados ao tema, utilizando os seguintes termos: “carcinoma papilífero de tireoide, ciclina D1, imunoistoquímica, diagnóstico, prognóstico.” com busca AND ou OR, considerando o título e/ou resumo e os escolhidos foram lidos na íntegra.
Resultados: A busca incluiu 77 artigos que foram compilados nesta revisão.
Conclusão: A ciclina D1 foi expressa na grande maioria dos CPT sendo a distribuição difusa predominante. Não houve correlação entre a expressão dela com qualquer característica clinicopatológica dos CPT
Detalhes do artigo

Este trabalho está licenciado sob uma licença Creative Commons Attribution 4.0 International License.
Referências
Instituto Nacional de Câncer do Ministério da Saúde. Rio de Janeiro: INCA; 2017.
Chitikova Z, Pusztaszeri M, Makhlouf AM, Berczy M, Delucinge-Vivier C, Triponez F, et al. Identification of new biomarkers for human papillary thyroid carcinoma employing NanoString analysis. Oncotarget. 2015;6(13):10978–93. Doi: 10.18632/oncotarget.3452
Cobin RH, Gharib H, Bergman DA, Clark OH, Cooper DS, Daniels GH, et al. AACE/AAES Medical/Surgical Guidelines for Clinical Practice: Management of Thyroid Carcinoma. Endocr Pract. 2001;7(3):202–20. Available in: 10.4158/EP.7.3.202.
Kim TH, Park YJ, Lim JÁ, Ahn HY, Lee EK, Kim KW, et al. The association of the BRAFV600E mutation with prognostic factors and poor clinical outcome in papillary thyroid cancer: A meta-analysis. Cancer. 2012;118(7):1764–73. Doi: 10.1002/cncr.26500
Bertagna F, Piccardo GTA, Giubbini R. Diagnostic and Clinical Significance of F-18-FDG-PET/CT Thyroid Incidentalomas. J Clin Endocrinol Metab. 2012;97(11):3866–75. Doi: 10.1210/jc.2012-2390
Barbet J, Campion L, Kraeber-Bodéré F, Chatal JF. Prognostic impact of serum calcitonin and carcinoembryonic antigen doubling-times in patients with medullary thyroid carcinoma. J Clin Endocrinol Metab. 2005;90(11):6077–84. Doi: 10.1210/jc.2005-0044
Giovanella L, Ceriani L, Maffioli M. Postsurgery serum thyroglobulin disappearance kinetic in patients with differentiated thyroid carcinoma. Head Neck. 2010;32(5):568–71. Doi: 10.1002/hed.21214
Giraudet AL, Ghulzan AA, Aupérin A, Leboulleux S, Chehboun A, Troalen F, et al. Progression of medullary thyroid carcinoma: assessment with calcitonin and carcinoembryonic antigen doubling times. Eur J Endocrinol. 2008;158(2):239–46. Doi: 10.1530/EJE-07-0667
Miyauchi A, Kudo T, Miya A, Kobayashi K, Takamura Y, Higashiyama T, et al. Prognostic Impact of Serum Thyroglobulin Doubling-Time Under Thyrotropin Suppression in Patients with Papillary Thyroid Carcinoma Who Underwent Total Thyroidectomy. Thyroid. 2011;21(7):707–16. Doi: 10.1089/thy.2010.0355
Pesutić-Pisac V, Punda A, Gluncic I, Bedekovic V, Kragic AP, Kunac N. Cyclin D1 and p27 expression as prognostic factor in papillary carcinoma of thyroid: association with clinicopathological parameters. Croat Med J. 2008;49:643-9. Doi: 10.3325/cmj.2008.5.643
Bhaijee F, Nikiforov YE. Molecular analysis of thyroid tumors. Mod Pathol. 2011;22:126-33. Doi: 10.1007/s12022-011-9170-y.
Seybt TP, Ramalingam P, Huang J, Looney SW, Reid MD. Cyclin D1 expression in benign and differentiated malignant tumors of the thyroid gland: diagnostic and biologic implications. Appl Immunohistochem Mol Morphol. 2012;20(2):124–30. Doi: 10.1097/PAI.0B013E31822D4783.
Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedus L, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. J Endocrinol Invest. 2016;16(3)468-75. Doi: 10.4158/EP.16.3.468
Tuttle RM. Papillary thyroid cancer. In: Post TW, editor. UpToDate. Waltham, MA: UpToDate Inc.; 2018. Available in: https://www.uptodate.com/contents/papillary-thyroid-cancer?source=bookmarks_widget#H13329960
Bibbins-Domingo K, Crossman DC, Curry SJ, Barry MJ, Dacidson KW, Doubeni CA, et al. Screening for thyroid cancer: US preventive services task force recommendation statement. JAMA. 2017;317(18):1882-87. DOI: 10.1001/jama.2017.4011
Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97(5):418-28. Doi: 10.1016/0002-9343(94)90321-2.
Perros P, Boelaert K, Colley S, Evans C, Evans RM, Ba GG, et al. Guidelines for the management of thyroid cancer. Clin Endocrinol. 2014;81(suppl 1):1-122. Doi: 10.1111/cen.12515.
Patel KN, Singh B. Genetic Considerations in Thyroid Cancer. Cancer Control. 2006;13(2):111–18. Doi: 10.1177/107327480601300205
Segev DL, Umbricht C, Zeiger MA. Molecular pathogenesis of thyroid cancer. Surg Oncol. 2003;12(2):69–90. Doi: 10.1016/s0960-7404(03)00037-9
Cao CD, Wémeau JL. Cancer de la Thyroïde. In: Traité de Médicine Akos. Paris: EMC (Elsevier Masson SAS); 2008. p. 3–0500. Doi: 10.1016/S1634-6939(20)64720-2
Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133. Doi: 10.1089/thy.2015.0020
Hundahl SA, Fleming ID, Fremger AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985-1995. Cancer. 1998;83(12):2638-48. Doi: 10.1002/(sici)1097-0142(19981215)83:12<2638::aid-cncr31>3.0.co;2-1
Rago T, Scutari M, Santini F, Loiacono V, Piaggi P, Coscio GD, et al. Real-time elastosonography: Useful tool for refining the presurgical diagnosis in thyroid nodules with indeterminate or nondiagnostic cytology. J Clin Endocrinol Metab. 2010;95(12):5274-80. Doi: 10.1210/jc.2010-0901
Sebag F, Vaillant-Lombard J, Berbis J, Griset V, Henry JF, Petit P, et al. Shear wave elastography: a new ultrasound imaging mode for the differential diagnosis of benign and malignant thyroid nodules. J Clin Endocrinol Metab. 2010;95(12):5281-8. Doi: 10.1210/jc.2010-0766.
Balasubramanian SP, Harrison BJ. Systematic review and meta-analysis of sentinel node biopsy in thyroid cancer. Br J Surg. 2011;98(3):334-44. Doi: 10.1002/bjs.7425
Hershman JM, Cheng S, Gianoukakis AG. Update in Thyroidology 2010. J Clin Endocrinol Metab. 2011;96(1):9-14. Doi: 10.1210/jc.2010-2350
Mekel M, Nucera C, Hodin RA, Parangi S. Surgical implications of B-RafV600E mutation in fine-needle aspiration of thyroid nodules. Am J Surg. 2010;200(1). Doi: 10.1016/j.amjsurg.2009.08.029.
Novoa E, Gurtler N, Arnoux A, Kraft M. Role of ultrasound-guided core-needle biopsy in the assessment of head and neck lesions: A meta-analysis and systematic review of the literature. Head Neck. 2012;34(10):1497-503. Doi: 10.1002/hed.21821
Webb RC, Howard RS, Stojadinovic A, Gaitonde DY, Wallace MK, Ahmed J, et al. The Utility of Serum Thyroglobulin Measurement at the Time of Remnant Ablation for Predicting Disease-Free Status in Patients with Differentiated Thyroid Cancer: A Meta-Analysis Involving 3947 Patients. J Clin Endocrinol Metab. 2012;97(8):2754–63. Doi: 10.1210/jc.2012-1533
Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384(9940):319-28. Doi: 10.1016/S0140-6736(14)60421-9.
Fallahi B, Beiki D, Takavar A, Fard-Esfahani A, Gilani KA, Saghari M, et al. Low versus high radioiodine dose in postoperative ablation of residual thyroid tissue in patients with differentiated thyroid carcinoma: A large randomized clinical trial. Nucl Med Commun. 2012;33(3):275-82. Doi: 10.1097/MNM.0b013e32834e306a.
Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. Lenvatinib versus Placebo in Radioiodine-Refractory Thyroid Cancer. N Engl J Med. 2015;372:621-30. Doi: 10.1056/NEJMoa1406470
Keating GM, Lyseng-Williamson KA, Frampton JE. Vandetanib: A guide to its use in advanced medullary thyroid cancer. BioDrugs. 2012;26(6):431-35. Doi: 10.1007/BF03261900.
Wells SA, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: A randomized, double-blind phase III trial. J Clin Oncol. 2012;30(2):134-41. Doi: 10.1200/JCO.2011.35.5040
Nagaiah G, Hossain A, Mooney CJ, Parmentier J, Remick SC. Anaplastic Thyroid Cancer: A Review of Epidemiology, Pathogenesis, and Treatment. J Oncol. 2011. Doi: 10.1155/2011/542358
Asiolis S, Erickson LA, Jebo TJ, Zhang J, Jin L, Thompson GB, et al. Papillary thyroid carcinoma with prominent hobnail features: A new aggressive variant of moderately differentiated papillary carcinoma. A clinicopathologic, immunohistochemical, and molecular study of eight cases. Am J Surg Pathol. 2010;34(1):44-52. Doi: 10.1097/PAS.0b013e3181c46677.
Faquin WC, Wong LQ, Afrogheh AH, Ali S, Bishop JÁ, Bongiovanni M, et al. Impact of reclassifying noninvasive follicular variant of papillary thyroid carcinoma on the risk of malignancy in The Bethesda System for Reporting Thyroid Cytopathology. Cancer Cytopathol. 2016;124(3):181–7. Doi: 10.1002/cncy.21631
Haugen BR. American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: What is new and what has changed? Cancer. 2015.
Xing M, Westra WH, Tufano RP, Cohen Y, Rosenbaum E, Phoden KJ, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005;90(12):6373-9. Doi: 10.1210/jc.2005-0987
Amin MB. AJCC Cancer Staging System, 8th Edition. Springer International Publishing; 2017.
Amin MB, Greene F, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93-9. Doi: 10.3322/caac.21388
Nixon IJ, Wang LY, Migliacci JC, Eskander A, Campbell MJ, Aniss A, et al. An international multi-institutional validation of age 55 years as a cutoff for risk stratification in the AJCC/UICC staging system for well-differentiated thyroid cancer. Thyroid. 2016;26(3):373-80. Doi: 10.1089/thy.2015.0315
Saito RF, Lana MV, Medramo RFV, Chammas R. Fundamentos de Oncologia Molecular. Atheneu: São Paulo; 2016.
Ghosh S, Srivastava S. Biomarkers in Cancer Screening and Early Detection. 1st ed. John Wiley & Sons, Inc; 2017.
Ward LS, Kloos RT. Molecular markers in the diagnosis of thyroid nodules. Arq Bras Endocrinol Metabol. 2013;57(2):89-97. Doi: 10.1590/S0004-27302013000200001.
Filetti S, Durante C, Leboulleux S, Locati LD, Newbold K, Papotti MG, et al. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:1856-83. Doi: 10.1093/annonc/mdz400.
Lloyd RV, Osamura RY, Kloppel G, Rosai J. WHO classification of tumours of endocrine organs. 4th ed. WHO Press; 2017.
De Matos LL, Giglio ABD, Matsubayashi CO, Farah M de L, Giglio AD, Pinhal MA da S. Expression of ck-19, galectin-3 and hbme-1 in the differentiation of thyroid lesions: Systematic review and diagnostic meta-analysis. Diagn Pathol. 2012;7(97):1596-7. Doi: 10.1186/1746-1596-7-97
Radu TG, Mogoanta L, Busuioc CJ, Stanescu C, Grosu F. Histological and immunohistochemical aspects of papillary thyroid cancer. Rom J Morphol Embryol. 2015;56(supl. 2):789-95.
Erickson LA, Jin L, Wolan PC, Tromson GB, Heerden JV, Lloyd RV. Expression of p27(kip1) and Ki-67 in benign and malignant thyroid tumors. Mod Pathol. 1998;11(2):169-74.
Wang S, Wuu J, Savas L, Patwardhan N, Khan A. The role of cell cycle regulatory proteins, cyclin D1, cyclin E, and p27 in thyroid carcinogenesis. Hum Pathol. 1998;29(11):1304–9. Doi: 10.1016/s0046-8177(98)90262-3.
Konturek A, Barczyński M, Nowak W, Richter P. Prognostic factors in differentiated thyroid cancer - A 20-year surgical outcome study. Langenbecks Arch Surg. 2012;397(5):809-15. Doi: 10.1007/s00423-011-0899-z
Alberts B. Molecular biology of the cell, 6th edition. 7th ed. Garland Publishing; 2015.
Gartner LP, Hiatt JL. Cell Biology and Histology. 7th ed. Wolters Klumer Health, S.A; 2015.
Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL. Cyclin D as a therapeutic target in cancer. Nat Rev Cancer. 2011;11(8):558–72. Doi: 10.1038/nrc3090.
Saiz AD, Olvera M, Rezk S, Florentine BA, McCourty A, Brynes RK. Immunohistochemical expression of cyclin D1, E2F-1, and Ki-67 in benign and malignant thyroid lesions. J Pathol. 2002;198(2):157–62. DOI: 10.1002/path.1185
Campbell NA. Campbell Biologie. 11ª ed. Pearson: Porto Alegre/RS; 2016.
Casimiro MC, Crosariol M, Loro E, Li Z, Pestell RG. Cyclins and Cell Cycle Control in Cancer and Disease. Genes Cancer. 2012;3(11-12):649-57. Doi: 10.1177/1947601913479022
Wagener C, Stocking C, Müller O. Cancer Signaling. 1st ed. Wiley-VCH Verlag GmbH & Co. KGaA; 2017.
Basolo F, Caligo MA, Pinchera A, Fedeli F, Baldanzi A, Micoli P, et al. Cyclin D1 overexpression in thyroid carcinomas: relation with clinicopathological parameters, retinoblastoma gene product, and Ki67 labeling index. Thyroid. 2000;10(9):741-6. Doi: 10.1089/thy.2000.10.741
Brzezianska E, Magierska AC, Sporny S, Pastuszak-Lewandoska D, Lewinski A. Assessment of cyclin D1 gene expression as a prognostic factor in benign and malignant thyroid lesions. Neuro Endocrinol Lett. 2007;28(4):341-50.
Donnellan R, Chetty R. Cyclin D1 and human neoplasia. J Clin Pathol Mol Pathol. 1998;51(1):1-7.
Lazzereschi D, Sambuco L, Scalzo CC, Ranieri A, Mincione G, Nardi F, et al. Cyclin D1 and Cyclin E expression in malignant thyroid cells and in human thyroid carcinomas. Int J Cancer. 1998;76(6):806–11. Doi: 10.1002/(sici)1097-0215(19980610)76:6<806::aid-ijc7>3.0.co;2-1
Qie S, Diehl JA. Cyclin D1, cancer progression, and opportunities in cancer treatment. J Mol Med. 2016;94(12):1313-26. Doi: 10.1007/s00109-016-1475-3
Hall M, Peters G. Genetic alterations of cyclins, cyclin-dependent kinases, and Cdk inhibitors in human cancer. Adv Cancer Res. 1996;68:67-108. Doi: 10.1016/s0065-230x(08)60352-8
Teshima M, Tokita K, Ryo E, Matsumoto F, Kondo M, Ikegami Y, et al. Clinical impact of a cytological screening system using cyclin D1 immunostaining and genomic analysis for the diagnosis of thyroid nodules. BMC Cancer. 2019;18;19(1):245. Doi: 10.1186/s12885-019-5452-4
Antonaci A, Consorti F, Mardente S, Natalizi S, Giovannone G, Rocca CD. Survivin and cyclin D1 are jointly expressed in thyroid papillary carcinoma and microcarcinoma. Oncol Rep. 2008;20(1):63-7.
Balta AZ, Filiz AI, Kurt Y, Sucullu I, Yucell E, Akin ML. Prognostic value of oncoprotein expressions in thyroid papillary carcinoma. Med Oncol. 2012;29(2):734–41. Doi: 10.1007/s12032-011-9969-x.
Jung CK, Kang YG, Bae JS, Lim DJ, Choi YJ, Lee KY. Unique patterns of tumor growth related with the risk of lymph node metastasis in papillary thyroid carcinoma. Mod Pathol. 2010;23(9):1201-8. Doi: 10.1038/modpathol.2010.116
Khoo MLC, Ezzat S, Freeman JL, Asa SL. Cyclin D1 protein expression predicts metastatic behavior in thyroid papillary microcarcinomas but is not associated with gene amplification. J Clin Endocrinol Metab. 2002;87(4):1810-3. Doi: 10.1210/jcem.87.4.8352.
Khoo MLC, Beasley NJP, Ezzat S, Freeman JL, Asa SL. Overexpression of cyclin D1 and underexpression of p27 predict lymph node metastases in papillary thyroid carcinoma. J Clin Endocrinol Metab. 2002;87(4):1814-18. Doi: 10.1210/jcem.87.4.8353
Kovacs GL, Stelkovics E, Krenacs L, Gonda G, Goth M, Kovacs L, et al. Low level of cyclin D1 protein expression in thyroid microcarcinomas from an autopsy series. Endocrine. 2005;26(1):41-4. Doi: 10.1385/ENDO:26:1:041
Kovacs GL, Stelkovics E, Krenacs L, Gonda G, Goth M, Kovacs L, et al. Low level of cyclin D1 protein expression in thyroid microcarcinomas from an autopsy series. Endocrine. 2005;26(1):41-4. Doi: 10.1385/ENDO:26:1:041
Muro-Cacho CA, Holt T, Klotch D, Mora L, Livingston S, Futran N. Cyclin D1 Expression as a Prognostic Parameter in Papillary Carcinoma of the Thyroid. Otolaryngol Head Neck Surg. 1999;120(2):200-7. Doi: 10.1016/S0194-5998(99)70407-9.
Lee SH, Lee JK, Jin SM, Lee KC, Sohn JH, Char SW, et al. Expression of cell-cycle regulators (cyclin D1, cyclin E, p27kip1, p57kip2) in papillary thyroid carcinoma. Otolaryngol Head Neck Surg. 2010;142(3):332-7. Doi: 10.1016/j.otohns.2009.10.050
Londero SC, Godballe C, Krogdahl A, Bastholt L, Specht L, Sorensen CH, et al. Papillary microcarcinoma of the thyroid gland: Is the immunohistochemical expression of cyclin D1 or galectin-3 in primary tumour an indicator of metastatic disease? Acta Oncol. 2008;47(3):451–7. Doi: 10.1080/02841860701630242
Bartolomei IJP, Ribas CAPM, Zella MAK, Waaga-Gasser AM, Gasser M, Czeczko NG, Nassif PAN. Immunohistochemical analysis of the cyclin D1 biomarker in papilliferous thyroid carcinomas and multinodular goiters. In: SciELO Preprints. 2022. Doi: 10.1590/SciELOPreprints.4150